People with penicillin allergies have been told they can have the Pfizer vaccine or any other Covid-19 vaccine. As the Medicines and Healthcare products Regulatory Agency MHRA has continued their close surveillance of the vaccine roll out they have now advised that individuals with a history of allergy or anaphylaxis to any food can receive any COVID-19 vaccine as long as they are not known to be allergic to any component excipient of the vaccine.

Penicillin Allergy Evaluations
Yes allergy to penicillins is not a contraindication to the PfizerBioNTech or AstraZeneca COVID-19 vaccine or Moderna vaccine.

I am allergic to penicillin can i have the astrazeneca covid vaccine. The Sun Online has contacted the MHRA for clarification on whether those with penicillin allergies are excluded from having the Covid jab. Severe allergies should be added to the possible side effects of AstraZenecas coronavirus vaccine after likely links were found to a number of. Generalised allergic reaction without anaphylaxis to one of the ingredients in the COVID-19 vaccine to be administered Pfizer-PEG or AstraZeneca-Polysorbate 80.
However Dr Pandit says that most medications or antihistamines used by those suffering from allergies have been found to be safe when used with the COVID-19 vaccine. Can you have the Covid vaccine if you are allergic to penicillin. If a person has had an allergic reaction to another vaccine this does not mean that they will also be allergic to the COVID-19 vaccine.
Last year I took my first penicillin vaccine. The agency also advises individuals with allergies to speak to their doctor before deciding on their suitability for vaccination but says that you may receive the vaccine even if you have an allergy. Currently the MRHA is advising anyone with a history of anaphylaxis or severe allergic reactions not to receive the Pfizer.
CDC recommends that people get vaccinated even if they have a history of severe allergic reactions not related to vaccines. If you have a history of immediate onset anaphylaxis to multiple classes of drugs or unexplained anaphylaxis please also refer to the additional information at. The only person who has a significant contrary indication to the current mRNA vaccines are those who have allergies to.
Allergy to penicillins is not a contraindication reason to withhold to the PfizerBioNTech or AstraZeneca COVID-19 vaccine or Moderna vaccine Covid vaccine. CDC says people with history of severe allergic reactions can get Covid-19 vaccine. People with a known PEG allergy or previous anaphylaxis to multiple drugs medications should see their clinical immunologyallergy specialist to assess and confirm their allergy.
Various Covid vaccine. Back in early December use of the Pfizer vaccine in people with a history of severe allergies was paused in the UK after two NHS workers who both had a history of allergies suffered a mild reaction to the vaccine. I cant say because I am a penicillin virgin.
The Sun Online has contacted the MHRA for clarification on whether those with penicillin allergies are excluded from having the Covid jab. Prior history of anaphylaxis to previous vaccines andor multiple drugs injectable andor oral where the ingredients PEG or polysorbate 80 may conceivably be the cause and have not been tolerated since on medication review. If You Are Allergic to Other Types of Vaccines.
The Oxford-AstraZeneca vaccine does not contain PEG 2000 so remains an alternative for people with a history of allergy to this ingredient2 However there is some cross-reactivity between PEG and polysorbate 80 an ingredient in the Oxford-AstraZeneca vaccine so evaluation by an allergy specialist may be advisable before vaccination in anyone with a suspected PEG allergy history14 Allergy is antigen specific although people with one drug allergy. The good doctor asked if I had any history of allergic reactions to penicillin. If You Have Allergies Not Related to Vaccines.
Your doctor will help you decide if it is safe for you to get vaccinated. If you have had an immediate allergic reactioneven if it was not severeto a vaccine or injectable therapy for another disease ask your doctor if you should get a COVID-19 vaccine. None of the currently approved UK COVID-19 vaccines.
STAFF REACTION Guidance for the vaccine was altered after two NHS staff members who received the jab last month had allergic reactions. It is very safe for them to do that Levine said.
Following fast on the heels of Pfizers announcement of its COVID-19 vaccine efficacy Moderna is also sharing positive results from its Phase 3 trial. Phase 3 AstraZeneca Coronavirus Vaccine Study Reopens at URMC Update November 23 2020.

Moderna Pfizer Biontech Vaccines Have Been Approved In Canada Here S What You Need To Know About Them Cbc News
AstraZeneca and Oxford University researchers expect to release full late-stage trial results by Christmas.
Astrazeneca covid vaccine phase 3 results. UPDATED ON OCT 14 2020 0225 PM IST The results of phase three of Oxford-AstraZeneca Covid-19 vaccine could be available by November-end or early. AstraZeneca today announced interim results of their phase 3 US study which indicate 79 overall efficacy of their vaccine against symptomatic COVID-19. Actual Study Start Date.
Actual Primary Completion Date. Oxford and AstraZeneca researchers present a pooled analysis in The Lancet of Phase 3 trials of a coronavirus vaccine resulting in an average efficacy of 704. Estimated Study Completion Date.
According to AstraZeneca late-stage trials of AZD1222 include a Phase III trial in Brazil 2000 participants ISRCTN89951424 a Phase IIbIII trial in the UK. CNN British drugmaker AstraZeneca said Monday it has started Phase 3 trials of its experimental coronavirus vaccine in the United States becoming. AstraZeneca which developed its vaccine with the University of Oxford became the first COVID-19 vaccine candidate to have efficacy results from phase 3.
A Phase III Randomized Double-blind Placebo-controlled Multicenter Study in Adults to Determine the Safety Efficacy and Immunogenicity of AZD1222 a Non-replicating ChAdOx1 Vector Vaccine for the Prevention of COVID-19. An independent committee overseeing AstraZenecas trial confirmed the results after 141 total participants in the study developed COVID-19 though the drugmaker didnt reveal how many cases occurred in the vaccine and placebo groups. AstraZeneca got very encouraging results from the Phase 3 Provent study for a new mix of antibodies monoclonal which could replace the Covid vaccineIts about a pre-exposure prophylaxis similar to the PrEP that is prescribed today to patients at risk of contracting HIV and which prevents contagion from Covid-19LAzd7442 this is its production name has proved effective in reducing the.
SeventyFour iStock. Adult participants in the phase 2 and 3 trials will be randomized to receive one or two doses of AZD1222 or a vaccine against meningococcal bacteria that will serve as the control. AstraZeneca issued updated phase three trial data for its Covid-19 vaccine on Wednesday after facing criticism earlier this week over a preliminary report from its US.
Another vaccine option in coming months. And Brazil show that the vaccine is up to 90 percent effective depending upon the dosing regimen. AstraZeneca released Phase 3 data showing promising results for a combination antibody therapy that prevents COVID-19 possibly opening the door.
AstraZeneca anticipates having Phase 3 trial results by the end of March potentially giving the US. 10500 participants 2020-001228. But in order to do so the company needs to smooth over its delivery delays with current commitments.
The first full peer-reviewed results of phase 3 trials of the COVID-19 vaccine developed by AstraZeneca and Oxford University show that it is safe and up to 90 effective in preventing infection supporting regulatory submissions for emergency use. The company now says. A multi-site Phase 3 clinical trial evaluating an investigational COVID-19 vaccine known as AZD1222 has begun.
We report on the first clinical efficacy results of ChAdOx1 nCoV-19 in a pooled analysis of phase 23 trials in the UK and Brazil and safety data from more than 20 000 participants enrolled across four clinical trials in the UK Brazil and South Africa. The trial will enroll approximately 30000 adult volunteers at 80 sites in the United States to evaluate if the candidate vaccine can prevent symptomatic coronavirus disease 2019 COVID-19. First peer-reviewed results of phase 3 human trials of Oxford coronavirus vaccine demonstrate efficacy University of Oxford.
AstraZeneca and the University of Oxford reported that preliminary results from studies being conducted in the UK.
Public Health Englands Immunisation Against Infectious Disease the Green book states that individuals with previous allergy to a medicine where the trigger has been identified including anaphylaxis can receive the AstraZeneca COVID-19 vaccine. Read on to find out why people with this allergy may want to hold off and for more on vaccination preparedness If You Have This Common Condition Tell Your Doctor Before the Vaccine.

Frequently Asked Questions About Covid 19 Vaccines National Foundation For Infectious Diseases
However your vaccination should be followed by a 30-minute observation period in a setting where personnel equipment and supplies are present to manage anaphylaxis if needed.
If i'm allergic to antibiotics can i take the covid vaccine. If you are allergic to any of the vaccine ingredients especially PEG polyethylene glycol or polysorbate you should not get the COVID vaccine. Experts say those are the people who would need to be monitored for 30 minutes after receiving the COIVD-19 vaccine and may benefit from seeing. Continue taking all your usual medications including aspirin and anti-inflammatory arthritis medication if they are part of your daily medication regimen.
Dr June Raine said growing evidence from a pool of at least 800000 people in the UK and probably 15 million people in the US who have had the vaccine has raised no additional concerns. If you are sick it would be best to. Previous advice from the MHRA said people with a range of allergies to food and medicines should not be given the Pfizer vaccine.
We do not know yet if taking other medications changes your risk of having an allergic reaction to the vaccine. However Dr Pandit says that most medications or antihistamines used by those suffering from allergies have been found to be safe when used with the COVID-19 vaccine. The Centers for Disease Control and Prevention CDC says that any patient with a history of allergic reaction to any vaccine or injectable therapy and anyone with a history of severe allergic.
Same goes for people who are HIV positive and take. However Winokur said people who have. Now the NHS is advising people who have severe allergies not to take the vaccine.
Is it OK to get the vaccine if Im. The first rounds of the vaccine in the UK. Vaccine is safe amongst those with food allergy and common allergic conditions like Asthma Allergic Rhinitis and Allergic Dermatitis.
Mbaeyi said people with other types of allergies to food latex pollen or other substances do not have to take special precautions and are recommended to receive the. If you have had a severe allergic reaction or an immediate allergic reactioneven if it was not severeto any ingredient in an mRNA COVID-19 vaccine you should not get either of the currently available mRNA COVID-19 vaccines Pfizer-BioNTech and Moderna. Additionally if you are taking antibiotics you can also still get vaccinated safely However if you can wait to get vaccinated you might want to do so.
According to experts if youre allergic to polyethylene glycol you should wait to get the COVID vaccine. This is true whether you are taking other medications or not. Are being given to the elderly and frontline workers and these initial vaccinations seemed to be going smoothly until two National Health Service NHS workers had an allergic reaction to the COVID vaccine.
The good doctor asked if I had any history of allergic reactions to penicillin. Even those who have allergies to penicillin antibiotics or types of medications are not at risk if they get a COVID-19 vaccine. If youve had a severe allergic reaction to another vaccine or injectable medication or have experienced anaphylaxis from any cause you can still receive the COVID-19 vaccine.
The only person who has a significant contrary indication to the current mRNA vaccines are those who have allergies to some component of that vaccine or have received that vaccine and had a. Anaphylaxis commonly occurs within 30 minutes of exposure and would. Only people who have an Anaphylaxis allergic reaction to any of the vaccine contents.
Mbaeyi said people with other types of allergies to food latex pollen or other substances do not have to take special precautions and are recommended to receive the. I cant say because I am a penicillin virgin. So if you have had an organ transplant and are taking immunosuppressant drugs or taking those drugs to treat an autoimmune disease or if youre taking certain cancer chemotherapies the immunosuppressive drug could decrease the efficacy of the vaccine says William Moss MD executive director of the International Vaccine Access Center at the Johns Hopkins Bloomberg School of Public Health.
Last year I took my first penicillin vaccine. If You Are Allergic to an Ingredient in a COVID-19 Vaccine. The Centers for Disease Control and Prevention CDC says that any patient with a history of allergic reaction to any vaccine or injectable therapy and anyone with a history of severe allergic.
People commonly report systemic side effects like fever headaches muscle aches joint pain chills and fatigue. Centers for Disease Control and Prevention has reported that allergic reactions to the COVID-19 vaccine can occur but they are.
Alaska Woman Has Allergic Reaction To Covid Vaccine Health Officials Track Safety Mpr News
Each year more than 165 million Americans get the flu shot.
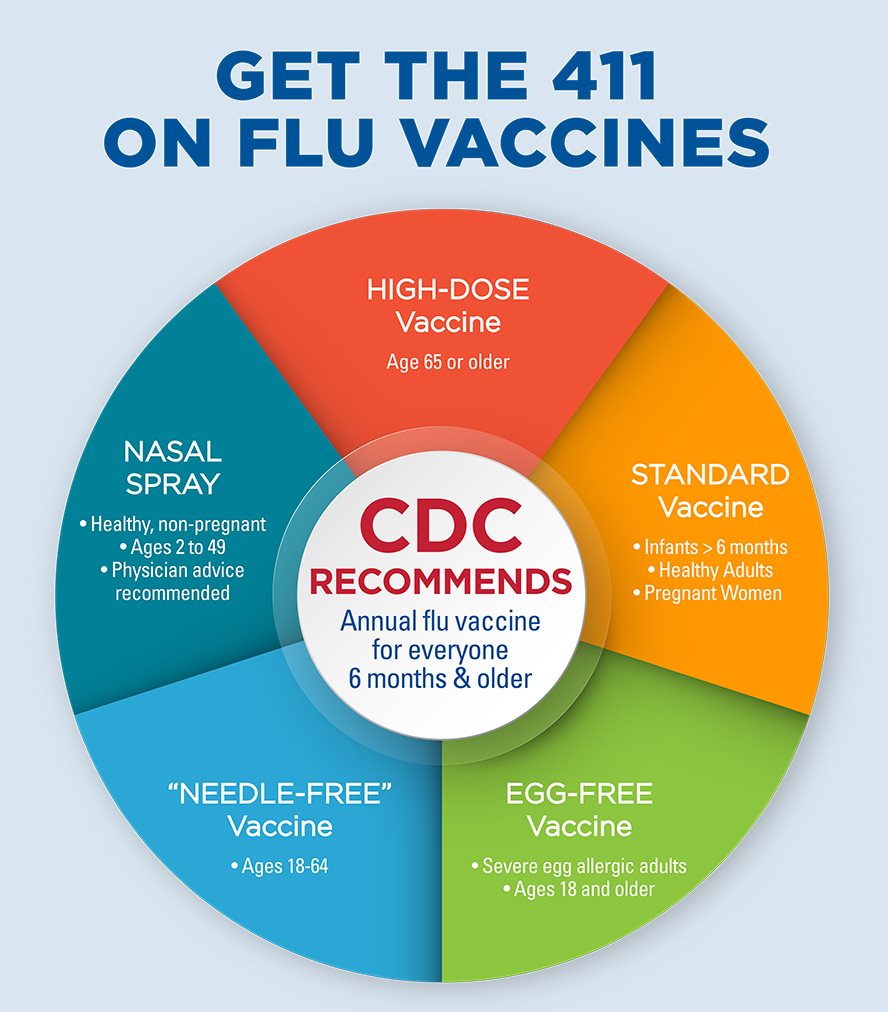
Allergic reaction to flu shot and covid vaccine. Some people especially after the second. People with an allergy to eggs are most at risk of having a severe allergic reaction but you may have a reaction to other components of the vaccine such as preservatives. These include pain at the injection site fatigue headache muscle pain chills joint pain and fever.
There were 85 reported deaths following influenza vaccination in 2017. 119 deaths in 2018. Those with mild allergic reactions to any other vaccine or who have food pet venom or latex allergies have the green light to get the COVID-19 vaccine but should be watched for 15 minutes.
If Im allergic to the flu shot could I have the same reaction to the COVID-19 vaccine Dr. In the Pfizer-BioNTech vaccine trials more than 77 of participants reported at least one systemic reaction. Murphy answers viewer COVID-19 questions 910.
Most people who get the vaccine experience some common side effects. People respond adversely to many vaccines including the flu vaccine which is incubated in chicken eggs for example. Other side effects may start within a few hours or up to 12 hours after the shot.
People who are allergic to eggs sometimes have an allergic reaction to the flu vaccine. Recognizing Allergic Reactions to a COVID-19 Vaccine Its important to distinguish side effects from the vaccine from allergic reactions to it. Severe allergic reactions to vaccines are extremely rare and were also rare during the PfizerBioNTech trial which excluded people with a history of anaphylaxis.
Allergic reactions arent a unique side effect of the COVID-19 vaccine Brown says. If you had an allergic reaction to the first dose of a COVID-19 vaccine the CDC doesnt recommend getting the second dose. Respiratory symptoms of a serious allergic reaction to the vaccine could include the sensation of your throat closing a high-pitched sound while breathing shortness of breath wheezing and coughing.
There is no known relationship between allergic reactions to insect stings and the COVID-19. 12 Hours After Vaccination. The main trigger for this severe reaction surprisingly is egg.
If You Are Allergic to an Ingredient in a COVID-19 Vaccine. The vaccine against the coronavirus that causes COVID-19 is not the same as the influenza vaccine. An allergy to the flu vaccine is concerning because it can lead to a potentially life-threatening reaction known as anaphylaxis.
They are not a reason to avoid the PfizerBioNTech AstraZeneca Moderna or Janssen COVID-19 vaccine. An immediate allergic reaction happens within 4 hours after getting vaccinated and could include symptoms such as hives swelling and wheezing respiratory distress. But overall having allergies doesnt exclude you from getting the vaccine.
Its recommended that if youve had an allergic reaction any of the ingredients in the vaccine you should not get it. Experts say that depends on the type of reaction you had. FluMist and most flu shots are manufactured using egg-based technology.
I want to get the COVID-19 vaccine but I fear another severe reaction. Many common side effects of vaccination such as localised pain and swelling at the site of injection or flu-like symptoms can be mistaken for allergy. I had a violent reaction to a flu shot in 1976 that continues to have an effect in my body today and I am 74 years old.
Anaphylaxis due to the COVID vaccine can also result in gastrointestinal symptoms. CDC says people with history of severe allergic reactions can get Covid-19 vaccine. Also had a allergic reaction to receiving my flu shot I would like to get the vaccination but im.
Could I have a reaction to the COVID vaccine shot The answer is no. Severe allergic reactions to COVID-19 vaccines are very rare. The risk of having a severe allergic reaction to any vaccine including the flu shot is 13 in a million.
A new CDC study published Wednesday found that people receiving Covid-19 vaccines experience anaphylaxis at a rate 10 times higher compared with the flu vaccine. The FDA has released data. And 203 deaths in 2019Between mid-December 2020 and April 23 2021 at which point between 95 million and 100 million Americans had received their COVID-19 shots there were 3544 reported.
Experts refer to severe allergic reactions as anaphylaxis. Most importantly out of more than 14 million administered COVID-19 vaccines there have been zero deaths associated with allergic reactions largely because anaphylaxis is completely treatable with epinephrine injections.
The PVTU working with UPMC Childrens Hospital of Pittsburgh is one of 100 study sites in the US. Each vaccine group will enroll about 25 people ages 18 through 55 years and approximately 25 people age 56 years and older.

Some Teens Volunteer For Covid Vaccine Trials To Get Their Lives Back The New York Times
In this region Moderna is recruiting participants for a site at the Childrens Hospital of Philadelphia.

How to enroll in covid vaccine trial. PITTSBURGH The University of Pittsburgh School of Medicine through the Pittsburgh Vaccine Trials Unit PVTU is one of twelve sites across the country selected to participate in a National Institute of Allergy and Infectious Diseases clinical trial in which fully vaccinated adults will receive a third booster dose of a COVID-19 vaccine. Study of COVID-19 Vaccines in Individuals With and Without Immune Deficiencies. By using a placebo medical researchers are able to understand if the study vaccine is effective in preventing COVID-19.
Parents with children ages 6 months to 11 years old listen up. In the case of the Covid-19 vaccine trials this would include tests to make sure the prospective volunteer hasnt already been exposed to the virus which would make them ineligible. Tests vaccines and treatments will help slow and possibly put an end to COVID-19 and the impact its had on the world and in our communities.
This is usually done over camera where audio-visual informed consent is taken. Vaccinating children as young as 6 months of age against COVID-19 may become the new front in the global pandemic fight if the vaccines prove to be safe and effective. Wed have to.
If youre willing your kids can participate in a clinical trial for the covid vaccine. Last week I got an offer to enroll my 14-month-old in a COVID vaccine trial for children writes Irin Carmon. On November 18 Pfizer and BioNTech announced that after conducting the primary efficacy analysis their mRNA-based COVID-19 vaccine met all of the studys.
Is It Safe to Enroll Your Kid in a COVID Vaccine Trial. Find out why these parents have Shubhangi Misra. Moderna whose vaccine currently is only available for adults began recruiting kids for its pediatric vaccine trial in March.
Right now scientists are working hard to. UC Health in Cincinnati is enrolling participants for clinical trials designed to evaluate the safety and effectiveness of COVID-19 vaccines. How to register for a COVID-19 vaccine in every Bay Area county Map shows everywhere you can get a COVID-19 test in the Bay Area Interactive.
Twelve to 20 weeks following their initial vaccination regimen participants will receive a single booster dose of the Moderna COVID-19 vaccine as part of the trial. Taliban Drags 21-Year-Old Woman Out. To evaluate the immune response to COVID-19 vaccination and any vaccine-associated adverse events that may be experienced in individuals with different immune disorders.
The trial will study the safety and immune response of a. As previously announced the Medical Center along with other select academic medical centers plans to test the COVID-19 vaccine on 200300 volunteers among healthy children aged 6 months to 11 years of age. This trial began July 27 2020 and completed enrollment of 46331 participants in January 2021.
Interested parties must complete a brief study and a. In-person visits at the NIH are optional. Understand the virus and how it works in the body.
The Phase 3 clinical trial was designed to determine if the Pfizer-BioNTech COVID-19 vaccine is safe and effective in preventing COVID-19 disease. The main objective for every clinical trial is safety Muller says. The Pittsburgh Vaccine Trials Unit PVTU has joined the pediatric COVID-19 Moderna KidCOVE vaccine trial and is enrolling children ages 6 months to 12 years.
To be a vaccination provider your health system or you as an independent provider are required to sign the CDC COVID-19 Vaccination Program Provider Agreement. You and your child will be asked to return to the study site up to six. Research in the form of clinical trials and studies can help make that happen as quickly as possible.
The volunteer is then tested to see whether he or she is eligible for the trial. You must be legally authorized in your jurisdiction to administer vaccines. All samples may be collected remotely.
My only worry was that I wouldnt make the appointment in time. Things to know before signing the agreement. Even in those trials that are in relatively late stages such as the COVID vaccine trials the most important assessment that we make is that patients are staying safe.
One such trial by the. Would you enroll your kid for Covid vaccine trial. COVID-19 vaccines are 100 free for the patient.
And Canada participating in the study which seeks to enroll 7050 children. Enrollment begins June 7 for children ages 511 for the Pfizer vaccine trial with Moderna trials expected to start in August.