The Oxford-AstraZeneca covid-19 vaccine is not linked to an increased risk of blood clots and is both safe and effective an investigation by the European Medicine Agency EMA has concluded1 The in-depth analysis of evidenceincluding laboratory results clinical reports autopsies and clinical trial datawas carried out after a small number of blood clot cases 37 were reported in. The expected benefits of the vaccines in preventing COVID-19 and serious complications associated with COVID-19 far.
Latest Results Put Oxford Astrazeneca Covid Vaccine Back On Track
The OxfordAstraZeneca COVID-19 vaccine codenamed AZD1222 and sold under the brand names Covishield and Vaxzevria among others is a viral vector vaccine for prevention of COVID-19Developed by Oxford University and AstraZeneca it is given by intramuscular injection using as a vector the modified chimpanzee adenovirus ChAdOx1.

Astrazeneca covid-19 vaccine clinical trial results. AstraZeneca has published the primary analysis results from the Phase III clinical trials of its Covid-19 vaccine in the UK Brazil and South Africa which showed that the vaccine is safe and effective in preventing Covid-19 more than 22 days after the first dose. SeventyFour iStock The first full peer-reviewed results of phase 3 trials of the COVID-19 vaccine developed by AstraZeneca and Oxford University show that it is safe and up to 90 effective in preventing infection supporting regulatory submissions for emergency use. The drug made from a combination of two antibodies was initially developed as a treatment for those who had already been exposed to the disease.
Currently there are no specific treatments available against COVID-19 and accelerated vaccine development is urgently needed. The clinical trials showed the product to be safe and well tolerated. Late Monday the Data and Safety Monitoring Board DSMB notified NIAID BARDA and AstraZeneca that it was concerned by information released by AstraZeneca on initial data from its COVID-19 vaccine clinical trial.
New vaccine efficacy results are reported now in The Lancet. The Covid-19 drug of AstraZeneca has showed positive results during its clinical trial of treatment for the coronavirus symptoms. Studies carried out in 2020 showed that the efficacy of the.
The high-level results from the phase 3 clinical trial called PROVENT were unveiled today. The Russian Direct Investment Fund RDIF has announced the results of a small clinical study that was conducted in the Republic of Azerbaijan. The drug was made from a combination of two antibodies which was initially developed as a treatment for those who had already been exposed to the virus.
The COVID-19 pandemic has caused major disruption to healthcare systems with significant socioeconomic impacts. NIAID Statement on AstraZeneca Vaccine. Update March 22 2021.
A safe and effective vaccine for COVID-19 prevention would have significant public health impact. Data are first Phase 3 trial results of a coronavirus vaccine to be published in peer-review literature Today University of Oxford and AstraZeneca researchers present a pooled analysis of Phase 3 trials of a vaccine against SARS-CoV-2 across two different dose regimens resulting in an average efficacy of 704. By NIHNational Institute of Allergy and Infectious Diseases March 22 2021 Results from a large clinical trial in the United States and South America indicate that AstraZenecas COVID-19 vaccine AZD1222 is well-tolerated and protects against symptomatic COVID-19 disease including severe disease or hospitalization.
We report on the first clinical efficacy results of ChAdOx1 nCoV-19 in a pooled analysis of phase 23 trials in the UK and Brazil and safety data from more than 20 000 participants enrolled across four clinical trials in the UK Brazil and South Africa. The clinical trial met its primary endpoint reducing the risk of symptomatic COVID-19 by 77 percent for at least three months when administered prior to infection compared to a placebo. Representational image Drug firm AstraZeneca on Friday announced positive results from a trial of a treatment for COVID-19 symptoms.
04 Feb 2021. The PROVENT pre-exposure prophylaxis trial showed AZD7442 could potentially provide long-lasting protection with demonstrated clinical trial success. Results from a large US clinical trial involving more than 32000 people and testing the AstraZenecaOxford COVID-19 vaccine candidate are finally available -.
COVID-19 vaccine trial within 48 hours after health officials publicly criticized the drugmaker for using outdated information to. AstraZeneca will publish up-to-date results from its major US. The Medicines and Healthcare products Regulation Authority MHRA has said that Following widespread use of these vaccines across the UK the vast majority of suspected adverse reaction reports so far confirm the safety profile seen in clinical trials It added.
Investigators of four randomised controlled trials conducted in the UK South Africa and Brazil report pooled results of an interim analysis of safety and efficacy against COVID-19 of the OxfordAstraZeneca chimpanzee adenovirus vectored vaccine ChAdOx1 nCoV-19 AZD1222 in adults aged 18 years and older. The DSMB expressed concern that AstraZeneca may have included outdated information from. The results from a trial that looked at how AstraZeneca and Sputnik Light doses reacted when combined.
2020 has been a difficult year for all but has seen 58 vaccines against severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 be developed and in clinical trials1with some vaccines reportedly having more than 90 efficacy against COVID-19 in clinical trials.
Astrazeneca S Covid 19 Vaccine Is 79 Percent Effective In U S Trial Science News

Astrazeneca Defends Covid 19 Vaccine After Us Agency Questions Trial Results
Astrazeneca Expects Covid 19 Vaccine Trial Results This Year Wsj
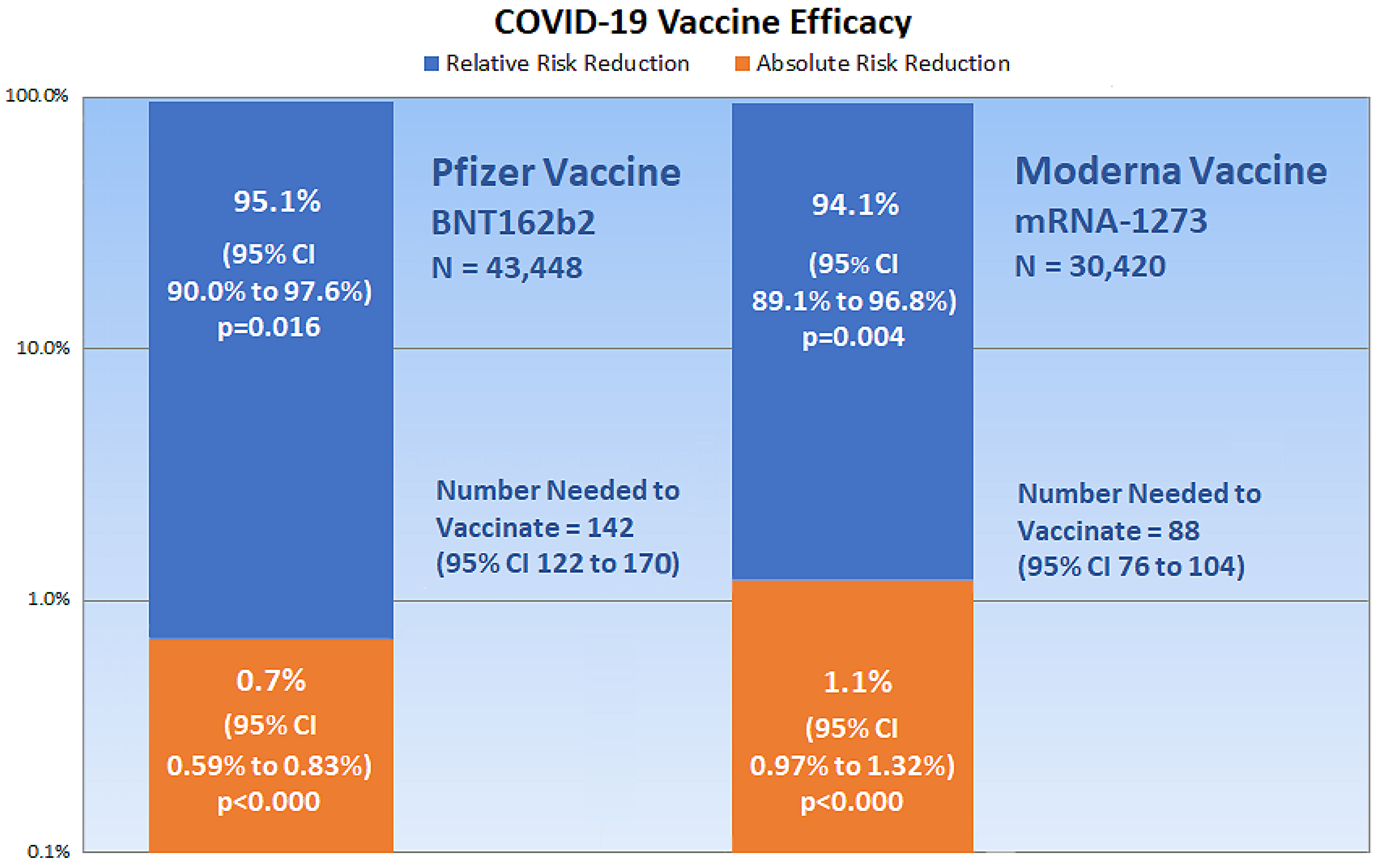
Medicina Free Full Text Outcome Reporting Bias In Covid 19 Mrna Vaccine Clinical Trials Html

Safety And Efficacy Of The Chadox1 Ncov 19 Vaccine Azd1222 Against Sars Cov 2 An Interim Analysis Of Four Randomised Controlled Trials In Brazil South Africa And The Uk The Lancet

What You Need To Know About Astrazeneca S Covid 19 Vaccine Science In Depth Reporting On Science And Technology Dw 18 03 2021
Covid Vaccine Us Trial Of Astrazeneca Jab Confirms Safety Bbc News

Doubts Raised Over Astrazeneca Oxford Vaccine Data Financial Times
.jpg)
Safety And Efficacy Of Pfizer And Astrazeneca Covid 19 Vaccines Even Better Than Clinical Trials Predicted
